Many healthcare professionals are encouraging people to get the recently updated COVID-19 vaccine this fall. The updated shots have been tweaked to protect against currently circulating coronavirus variants.
Since the COVID-19 vaccines were first rolled out in 2020, they have been at different stages of emergency use authorization (EUA) and U.S. Food and Drug Administration (FDA) approval, and it's been difficult for some to keep track of where they all stand.
VERIFY reader Migs asked if the updated COVID-19 vaccines have been fully approved by the FDA.
THE QUESTION
Are the updated COVID-19 vaccines fully approved by the FDA?
THE SOURCES
THE ANSWER
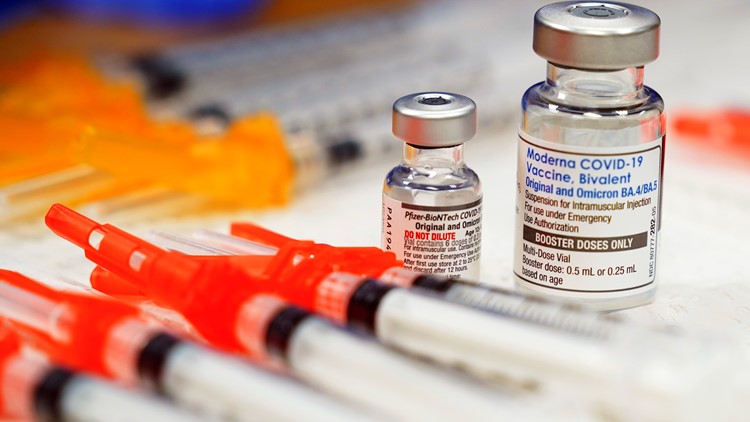
The updated Pfizer and Moderna mRNA COVID-19 vaccines are fully approved by the FDA for people age 12 and older. Both vaccines are also authorized for emergency use for children ages 6 months to 11 years old.
WHAT WE FOUND
The Food and Drug Administration (FDA) fully approved the updated Pfizer and Moderna mRNA COVID-19 vaccines for people age 12 and older on Sept. 11. They’re also authorized for emergency use for children ages 6 months to 11 years old.
The FDA also authorized the updated Novavax COVID-19 vaccine for emergency use in people 12 years of age and older. All of these vaccines have been updated to protect against the omicron variant XBB.1.5.
Emergency use authorization vs. full approval
The process for issuing an emergency use authorization (EUA) is different from FDA approval. Yale Medicine says it’s the job of the FDA to ensure medical products, such as drugs and vaccines, meet rigorous safety and efficacy standards in a process that can typically take years for what’s called “full approval.”
An emergency use authorization (EUA) is a tool the FDA used to expedite the availability of COVID-19 vaccines during the coronavirus pandemic. An EUA can only be granted when no adequate, approved, available alternatives exist, and when the known and potential benefits outweigh the potential risks, according to Yale Medicine.
The path to full FDA approval requires additional clinical data and information about the vaccine maker’s processes and facilities. This is used to determine whether the vaccine is safe and effective for its intended use and to make sure the benefits of the vaccine outweigh its risks when used according to its approved labeling, the FDA explains on its website.
Updated Pfizer and Moderna mRNA COVID-19 vaccines
The updated Pfizer and Moderna COVID-19 vaccines received full FDA approval for people age 12 and older on Sept. 11, 2023. Both vaccines are also authorized for emergency use for children ages 6 months to 11 years old.
Starting at age 5, the FDA said that most people can get a single dose of the updated Pfizer or Moderna vaccines even if they’ve never had a prior COVID-19 shot. Younger children might need additional doses depending on their history of COVID-19 infections and vaccinations.
Updated Novavax COVID-19 vaccine
The FDA also authorized the updated Novavax COVID-19 vaccine for emergency use in people 12 years of age and older on Oct. 3, 2023. Novavax makes a protein-based vaccine mixed with an immune-boosting chemical, which is a different technology than the mRNA vaccines made by Pfizer and Moderna.
Anyone age 12 and older who was previously vaccinated with a COVID-19 vaccine — and has not already received an updated mRNA COVID-19 vaccine — can get a single dose of the reformulated Novavax shot. The FDA says that unvaccinated people are eligible to receive two updated Novavax doses.
“The public can be assured that these updated vaccines have met the agency’s rigorous scientific standards for safety, effectiveness and manufacturing quality,” said Peter Marks, M.D., Ph.D., director of the FDA’s Center for Biologics Evaluation and Research. “We very much encourage those who are eligible to consider getting vaccinated.”
Where to find the updated vaccines
VERIFY found that the updated vaccine versions are free through private insurance or Medicare. The CDC’s Bridge Access Program also temporarily provides free shots to the uninsured or underinsured.
Vaccines.gov has information about where to find vaccines.
The Associated Press contributed to this report.
This story is also available in Spanish / Lee este artículo también en español: Las vacunas actualizadas de COVID de Pfizer y Moderna están completamente aprobadas para personas de 12 años en adelante