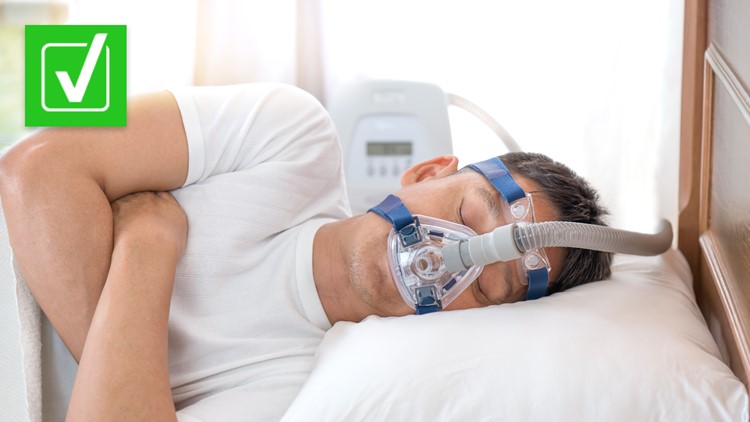
Sleep apnea is a medical condition that affects an estimated 22 million Americans. Typical symptoms of sleep apnea include heavy snoring, excessive daytime sleepiness or fatigue, and difficulty with concentration or memory, according to the American Sleep Apnea Association (ASAA). If untreated, sleep apnea can lead to serious health issues like heart disease, high blood pressure, stroke, depression and diabetes.
The ASAA says the most common treatment for moderate and severe sleep apnea is the nightly use of a continuous positive air pressure (CPAP) machine. In April 2021, CPAP machine manufacturer Philips Respironics warned of potential health risks related to the polyester-based polyurethane (PE-PUR) sound abatement foam used in some of its CPAP machines, bi-level positive airway pressure (BiPAP) devices and mechanical ventilators.
According to the U.S. Food and Drug Administration (FDA), the PE-PUR foam used in the Philips machines may break down and potentially enter the device’s air pathway. If this occurs, the FDA says black debris from the foam or certain chemicals may be inhaled or swallowed by the person using the device, which could result in serious, life-threatening injuries.
Several VERIFY viewers have sent us messages asking if the machines have been recalled and if any lawsuits have been filed against Philips. Here’s what we know.
THE QUESTION
Has Philips recalled some of its ventilators, CPAP and BiPAP machines?
THE SOURCES
THE ANSWER
Yes, Philips has recalled some of its ventilators, CPAP and BiPAP machines. Customers affected by the recall can register online or call 877-907-7508 to get their device repaired or replaced.
WHAT WE FOUND
On June 14, 2021, Philips Respironics issued a voluntary recall of several of its ventilators, CPAP and BiPAP machines in connection with the potential health risks detailed in the company’s April 2021 product warning. The majority of the affected devices, which were manufactured between 2009 and April 26, 2021, are in the first-generation DreamStation product family. Click here for a list of the recalled machines.
Philips submitted 30 medical device reports (MDRs) to the FDA between 2014 and April 2021 that were related to the PE-PUR foam breakdown, or degradation, in the machines. According to the FDA, it has received over 3,000 MDRs related to the foam breakdown since Philips’ April 2021 warning. The FDA says a wide range of adverse events have been reported to the agency, including cancer, pneumonia, asthma, other respiratory problems, infection, headache, cough, dyspnea (difficulty breathing), dizziness, nodules and chest pain.
If your device was affected by the recall, the FDA recommends talking to your health care provider first to decide if the plan for your care and treatment should change as a result of the recall. The FDA says your health care provider may advise you to stop using the recalled device, to use another similar device that has not been recalled, to continue using the recalled device or to use other sleep apnea treatments.
Customers affected by the recall can register online or call 877-907-7508 to get a repaired or replacement device from Philips. On Oct. 18, 2021, Philips reported that the company had produced “approximately 750,000 repair kits and replacement devices, of which more than 250,000 have reached customers.” On its website, Philips says the replacement process will take approximately 12 months to complete.
After registering to get a new machine, Ronald V. Miller Jr., an attorney at Miller & Zois in Maryland, says people should contact a lawyer if they can attribute a serious injury to their CPAP machine usage.
Over 100 lawsuits have already been filed against Philips in relation to the recall and were consolidated into a multidistrict litigation (MDL) on Oct. 8, 2021, by the United States Judicial Panel on Multidistrict Litigation. Miller told VERIFY MDLs are similar to class action lawsuits and says “it may take years to unravel all the potential harms caused by this defective PE-PUR foam.”
VERIFY reached out to Philips for comment.
More from VERIFY: Yes, the Old Navy class action settlement is real